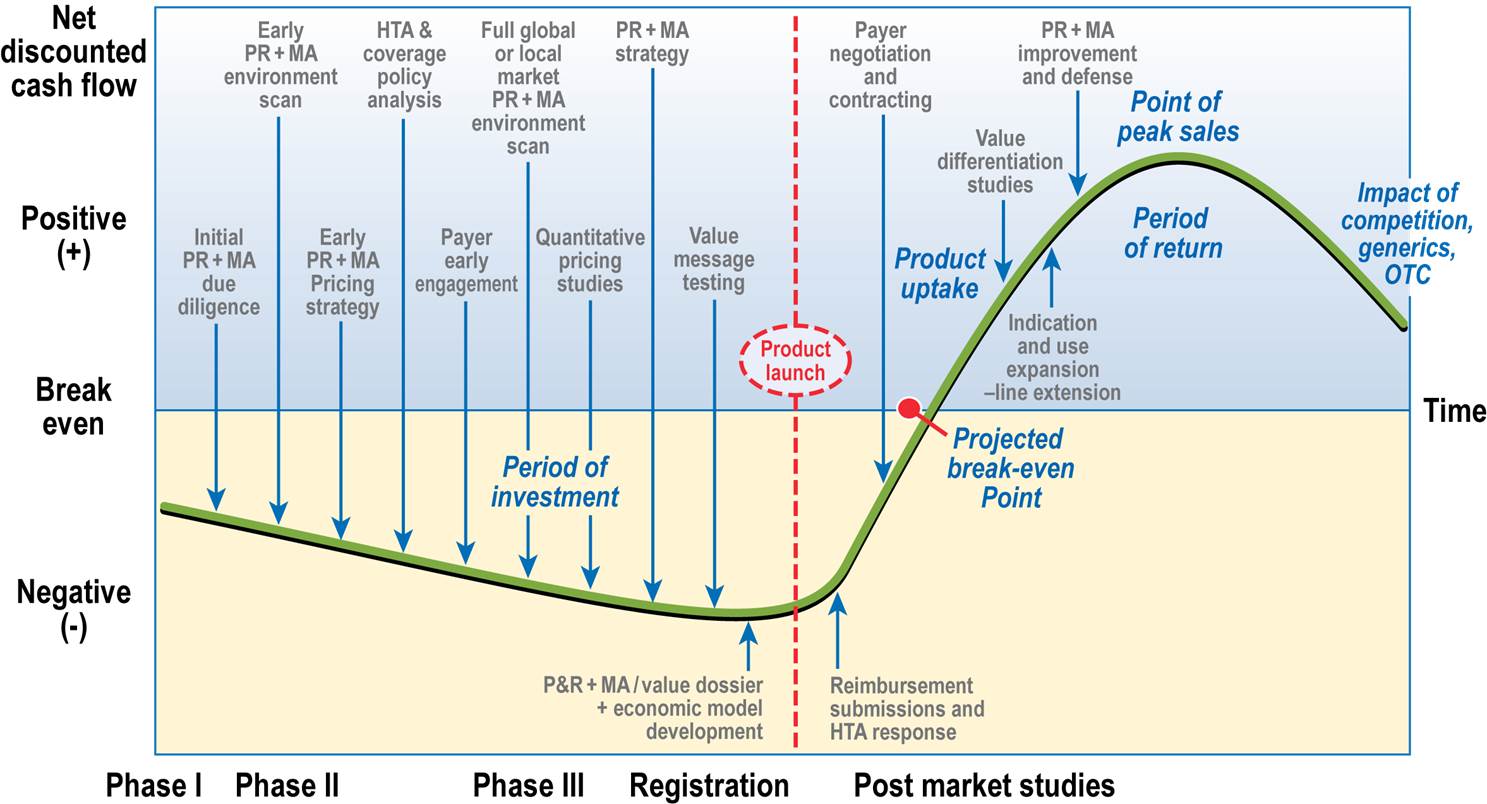
At every stage of your product’s lifecycle, we can help you develop a strategic roadmap to effectively demonstrate and communicate your product’s value. For clinical and commercial teams alike, we provide extensive research experience, industry and regulatory knowledge, and evidence-based tools to help commercialize your product.
To determine your best commercialization strategy, we can help you develop and execute a plan that includes:
- Environmental Scans: Commercial, clinical, payer strategy, and health economics
- Literature Reviews: Disease, burden of illness, treatment pathway, epidemiology and unmet need
- A Gap Analysis
- Target Product Profile (TPP) Assessment
- A Strengths, Weaknesses, Opportunities & Threats (SWOT) Analysis
- Proven and Projected Value Assessments: Clinical, economic, PRO/QoL value
- A Roadmap: Tactical project and study recommendations for filling the gaps
Market Access Planning Across the Product Lifecycle
Although it is never too late to develop a market access strategy, for the best positioning of your product, you should begin in Phase 2 of clinical development. Tasks to support the strategy should include the following activities.