Economic Qualitative Inquiry, Policy, and Access
Our focused research will enable you to demonstrate your products’ value to patients, physicians, caregivers, payers, and policymakers.
With so many public, private, and non-traditional payers and their ever-changing priorities, how do you keep up? But, given their role in your success, how do you not?
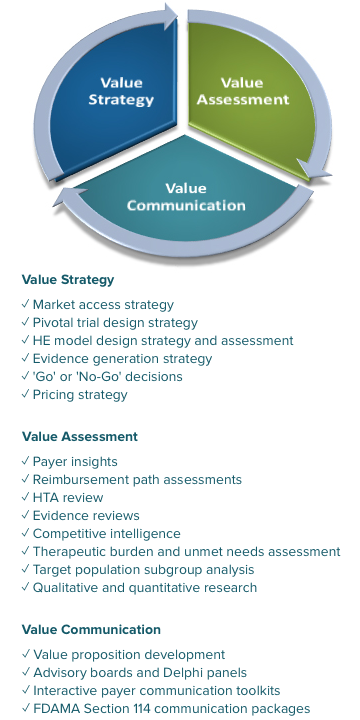
We can help you navigate the pricing and reimbursement bodies that ultimately determine the accessibility of your product. By engaging with national, regional, and local payers, payer decision-makers, and HTA agencies (e.g., private insurers, CMS, CADTH, NICE, IQWiG, HAS) about your new product, we can help you understand the clinical and economic evidence they will expect to see for your product to gain reimbursement.
Let us help you answer critical questions about:
- Payer perspectives and value drivers
- Price value mapping
- Critical pathway and landscape assessments
- Reimbursement issues to address in your value arguments
- Treatment attributes that influence pricing, reimbursement, and access decisions
- Evidence gaps and approaches to fill them
- Health equity and addressing disparities in marginalized populations
Advisory Panels
We have a global network of payers on our payer advisory panel, including a current member of NICE’s appraisal committee, the Chief Medical Officer of a major private US insurer, and the Pharmacy Director for a large national PBM.
Depending on your particular needs, we select the right panelists and appropriate methodology—including advisory panels, focus groups, interviews, surveys, Delphi panels, and mock pharmacy and therapeutics committees—to gain the most useful insights about your product, its competitors, and the current and future landscape. We also can assemble payer education boards to address negative coverage policies in the US and disease area concerns worldwide.
To get a well-rounded view of your products, we can also conduct clinician interviews and create advisory boards of different stakeholder groups to inform your product strategy, positioning, and value messaging.
Interactive Communication Toolkits
Your field-based staff and local affiliates need to clearly communicate to payers, prescribers, and patient advocates the unique value provided by your products. Toolkit projects include customizable slide decks typically with the following sections:
- Executive summary: Key product value messages and links to key background documents and environmental challenges
- Disease burden and unmet need summary
- Policy and environmental issues: current and after launch that impact your product’s market access
- Product value summary: Clinical, economic, humanistic, and comparative value
- Top-line objection handling
- In the US, Product information in accordance with Section 114 of the 1997 Food and Drug Administration Modernization Act
- Summaries of relevant studies including clinical trials and HEOR research
The toolkits are often developed with input from your local affiliates and validated with payer decision-makers in representative market(s) of interest. Typical audiences for these slide sets include stakeholders including national, regional, and local payers, prescribers, and patient advocates.
Field Testing Health Economics Models with Payers and Payer Advisors
Cost-effectiveness and budget-impact modeling play an integral part in payer decision-making. We can take your model to payers and payer advisors around the globe to ensure that input parameters, patient populations, and results from your modeling efforts are consistent with payer and payer advisor expectations prior to formal evaluations.