RTI Health Solutions is the place you turn when you need substantiated, authoritative evidence and advice. We are scientists first—academically trained, credentialed researchers who know how and when to apply the appropriate research methodologies to your real-world applications and situations. We care about doing what’s right and what’s relevant, so you can make good, safe, and timely decisions about your pharmaceutical, medical technology, or diagnostic products.
Multinational & Multidisciplinary: A global perspective
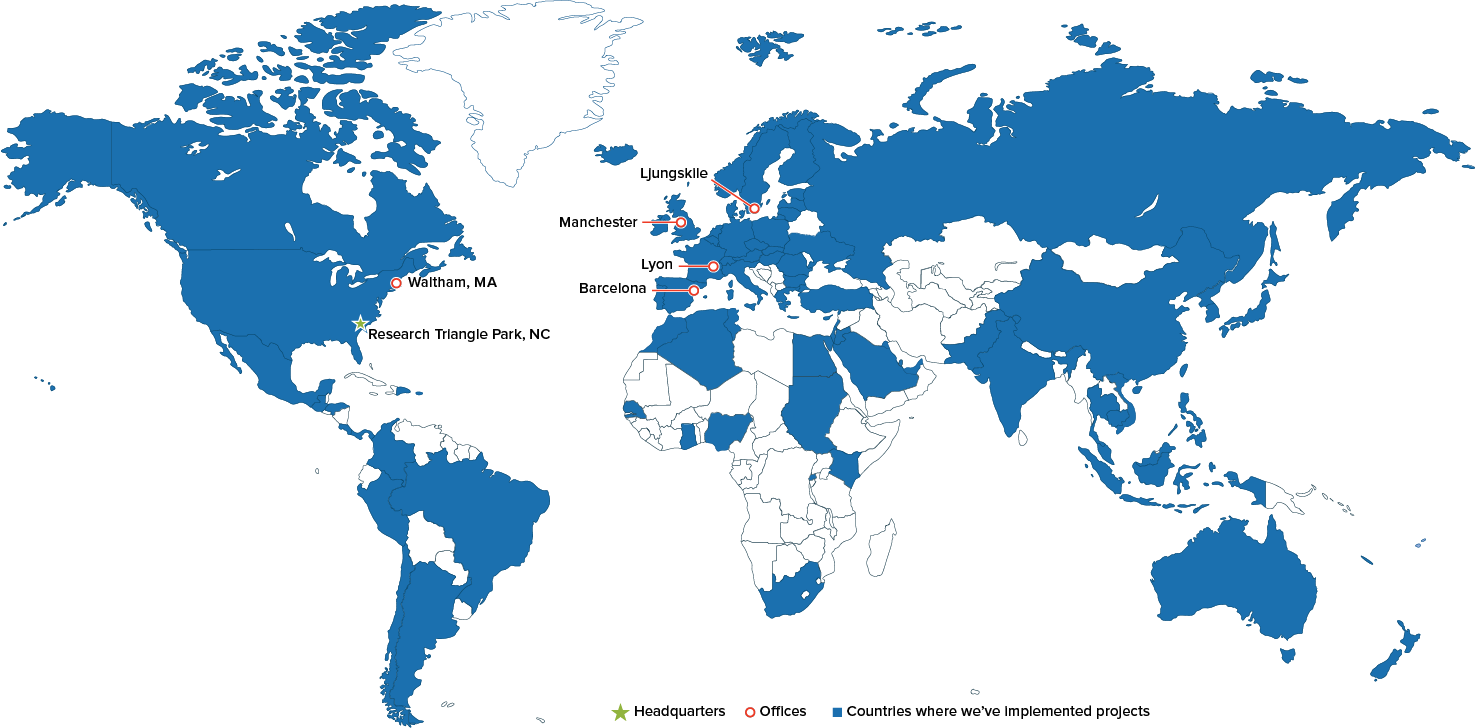
Located across the US and Europe, our staff have led projects in approximately 80 countries. Their experience is diverse; encompasses economics, public health, psychology, epidemiology, statistics, engineering, and other disciplines; and spans both developed and emerging markets, so we can help you with an array of projects in any region of interest. (Click here or the map to see a larger version.)
Expertise: Access to key thought leaders
We attract and retain top talent across each of our practice areas, so you always have access to key thought leaders in drug development, commercialization, and safety. Over 80% of our researchers hold master's or doctorate degrees. Nearly all are published in their fields, many serve as peer-reviewers and editors for research journals, and several maintain university and industry advisory appointments. Recognized for their mastery of essentials as well as cutting-edge methods, our experts are often asked to teach short courses and give presentations at conferences and industry meetings. Visit our webinar page to see a sampling of topics presented by our researchers.
Staff Stability: The retention connection
Our low turnover rates allow us to maintain stable project teams from inception to completion of projects, even on multi-year studies, and provide you with research continuity as your product goes through its development lifecycle. In our collaborative work environment, researchers take ownership of their work and find their jobs socially important and professionally rewarding—key contributors to our high employee retention rate.
Professional Affiliations: Enhancing our expertise and leadership
Because of their excellent reputation and professional associations, our experts are frequently called upon to participate on advisory committees and review boards. Through this work, they stay abreast of the latest industry trends and changes and they have a proactive role in guiding and shaping the industries we serve. Our affiliations with professional associations include the International Society for Pharmacoepidemiology (ISPE), ISPOR—The Professional Society for Health Economics and Outcomes Research, and International Society for Quality of Life Research (ISOQOL). Several staff also maintain academic appointments with a variety of top universities.
Independent Non-Profit Status: Results you can trust
As the only independent, non-profit organization in the industry, our primary objective is to provide the highest quality research and consulting services to benefit patients and improve public health. Our researchers believe what they do matters, and their commitment to science, impartiality, and professional reputation results in unbiased research that you can depend on to make better decisions.
Quality Assurance: Sharing your passion for excellence
Our standards of professional performance are enhanced by having an independent Quality office. The Quality team supports staff on regulations, guidelines, and the implementation of standard operating procedures and quality control processes that result in high-quality processes and deliverables. All deliverables also go through a rigorous independent editing process.
Your Partner in Publication
Meaningful research calls for communicating the results to the scientific community. We have a dedicated team of medical writers to support our research partners with Publication Management and Medical Writing.
Take a look at our knowledgebase archives to see easy-to-understand infographics that show insights and key tips for successful projects - from the experts at RTI Health Solutions.
View Knowledgebases Page