 Launch of the SIGMA Consortium heralds a new era of collaboration to better understand safety and effectiveness of medical interventions. Research institutions compete for resources to carry out research. SIGMA aims to address this challenge by integrating expertise and data from multiple European Research Centres. The new collaborative research initiative, designed to be the best ‘go-to’ organisation for comprehensive and rigorous methodological research, is being launched to enable multi-country studies using common analyses to study responses to treatments in large populations.
Launch of the SIGMA Consortium heralds a new era of collaboration to better understand safety and effectiveness of medical interventions. Research institutions compete for resources to carry out research. SIGMA aims to address this challenge by integrating expertise and data from multiple European Research Centres. The new collaborative research initiative, designed to be the best ‘go-to’ organisation for comprehensive and rigorous methodological research, is being launched to enable multi-country studies using common analyses to study responses to treatments in large populations.
SIGMA executive committee start up members Professor Ron Herings (Netherlands) and Dr. Susana Perez-Gutthann (Spain) said “The benefits and risks of treatments are of concern to every citizen. Our objective is to clarify and quantify these treatment effects for the citizens of Europe.”
The SIGMA Consortium’s VISION is to provide best evidence on the use, benefits and harms of medications, vaccines and devices for the betterment of public health and patient care. Its MISSION is to provide trusted pharmacoepidemiologic and real-world evidence (PE/RWE) research through a European federated professional network of excellence based on the ENCePP Code of Conduct. The SIGMA consortium welcomes collaborations with other groups and networks.
The SIGMA Consortium is formed by a group of leading research institutions and Universities with extensive expertise and experience in the conduct of pharmacoepidemiologic and real-world evidence research:
| Country | Institution | Lead |
|---|---|---|
|
Denmark |
Aarhus University, Department of Clinical Epidemiology | Prof. Vera Ehrenstein |
|
Denmark |
University of Southern Denmark, Department of Clinical Pharmacology and Pharmacy | Prof. Jesper Hallas, FISPE |
|
France |
University of Bordeaux’s Bordeaux PharmacoEpi Platform | Prof. Nicholas Moore, FISPE |
|
Germany |
Leibniz-Institut für Präventionsforschung und Epidemiologie – BIPS GmbH | Prof. Ulrike Haug |
|
Italy |
Agenzia Regionale di Sanità (ARS Toscana) | Dr. Rosa Gini |
|
Netherlands |
PHARMO Institute for Drug Outcomes Research | Prof. Ron Herings, FISPE |
|
Netherlands |
University Medical Centre Utrecht (UMCU) | Prof. Miriam Sturkenboom, FISPE |
|
Netherlands |
Utrecht University Division of Pharmacoepidemiology & Clinical Pharmacology | Prof. Olaf Klungel, FISPE |
|
Spain |
Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol) | Dr. Maria Giner |
|
Spain/USA |
RTI Health Solutions | Dr. Susana Perez-Gutthann, FISPE |
|
United Kingdom |
University of Dundee, Scotland | Prof. Thomas MacDonald, FISPE |
SIGMA Model of collaboration:
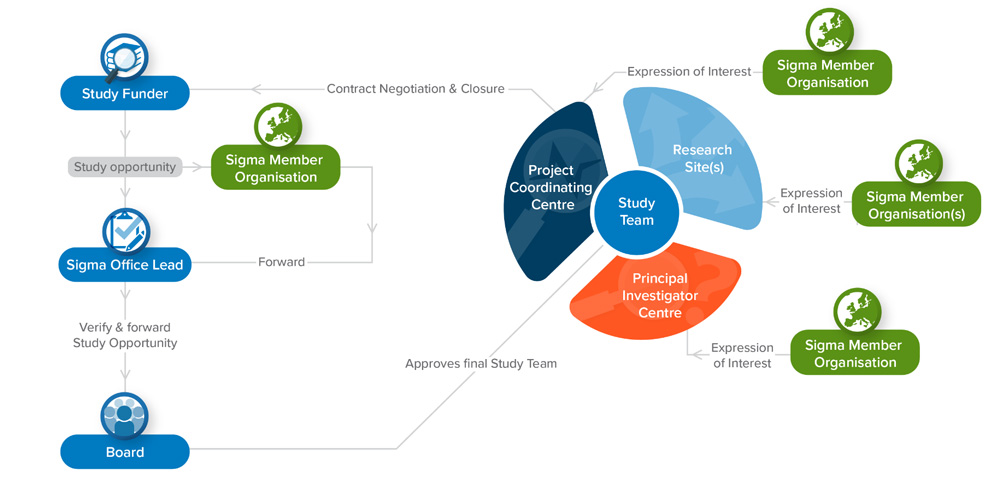
Adapted from VAC4EU (https://vac4eu.org/decision-making-and-roles-in-the-conduct-of-studies/)
Study funder institutions can contact the Sigma Consortium Office or a Sigma member organization. The request will be distributed to all Sigma members to express interest and availability to participate in the study in the roles of Coordinating Centre, Principal Investigator Centre and/or Research Site.
For more information, please contact
Josine Kuiper or Carla Franzoni at the SIGMA Office (info@sigmaconsortium.eu)
